Introduction:
The delayed platelet engraftment is a common complication in allogeneic hematopoietic stem cell transplantation (allo-HSCT), which elevates treatment-related mortality and impairs quality of life. Although some studies reported that platelet engraftment after allo-HSCT was promoted by thrombopoietin-receptor agonists (TPO-RAs), the recommended detailed TPO-RA administration is still lacking. This prospective, single-center, investigator-initiated pilot study aims to explore the efficacy and safety of herombopag, a second-generation TPO-RA, to stimulate platelet engraftment in haploidentical allo-HSCT (haplo-HSCT).
Methods:
A total of 36 patients (median age 38; range 15-59 years) with anaplastic anemia, myelodysplastic syndrome, acute leukemia, granulocytic sarcoma, or primary hemophagocytic lymphohistiocytosis were enrolled between November 2021 and June 2023. All patients received haplo-HSCT and post-transplant cyclophosphamide for acute graft-versus-host disease (aGVHD) prophylaxis. Patients received herombopag from day+5 post-HSCT until platelet>100,000/µL, disease progression, intolerable toxicity, or withdrawal of informed consent. The dose of herombopag was 5 mg/day initially and reduced to 2.5 mg/day when platelet>75,000/µL. Complete platelet engraftment (CPE) was defined as a platelet count exceeding 50,000/μl for seven consecutive days without transfusion. Partial platelet engraftment (PPE) was defined as a platelet count exceeding 20,000/μl for seven consecutive days without transfusion. The objective response included both CPE and PPE. The primary endpoint was the 21-day cumulative incidence and 28-day cumulative incidence of CPE and objective response after haplo-HSCT. The secondary endpoints were time to CPE, time to objective response, platelet transfusion units, aGVHD, relapse, non-relapse mortality, and safety.
Results:
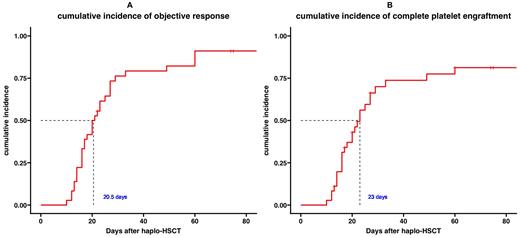
The patients received herombopag treatment for a median of 15 days. The 21-day cumulative incidence of CPE was 55.6%, and that of objective response was 77.8%. The 28-day cumulative incidence of CPE was 69.4%, and that of objective response was 80.6%. The median time to objective response was 20.5 (95%CI 17-27) days. The median time to CPE was 23 (95%CI 10-33) days. The mean units of platelet transfusions required within 30 days after HSCT was 6.7. The median follow-up time was 12.1 months. The one-year overall survival rate was 85.3% (95%CI 72.8%-99.8%). Two patients died of aGVHD and two patients died of lung infection. Four Patients (11.1%) experienced molecular relapse. The cumulative incidence of grade III-IV aGVHD was 16.7%. Furthermore, cytomegalovirus reactivation occurred in 13 patients (36.1%). The most common herombopag-related adverse events were hypomagnesemia (61.1%), increased GGT (52.8%), increased ALT (38.9%), and increased bilirubin (19.4%). No grade III/IV adverse events were observed.
Conclusion:
Herombopag significantly stimulates platelet engraftment in haplo-HSCT patients and has a favorable safety profile. Further research with a larger sample size and longer follow-up period is required to confirm its optimal use.
OffLabel Disclosure:
No relevant conflicts of interest to declare.
This is a prospective, single-center, investigator-initiated pilot study, aiming to improve platelet engraftment after haplo-HSCT.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal